Have you ever heard that water can boil and freeze at the same time? It sounds impossible, but it’s a real scientific phenomenon known as the Triple Point. This fascinating concept in physics occurs under specific conditions and is not just limited to water—it applies to many other substances too.
In this article, we will explore:
✅ What the Triple Point is and how it works.
✅ The science behind water boiling and freezing at the same time.
✅ Real-life applications of the Triple Point.
✅ How you can experiment with this concept at home.✅ What the Triple Point is and how it works.
✅ The science behind water boiling and freezing at the same time.
✅ Real-life applications of the Triple Point.
✅ How you can experiment with this concept at home.
Let’s dive deep into this mind-blowing fact!
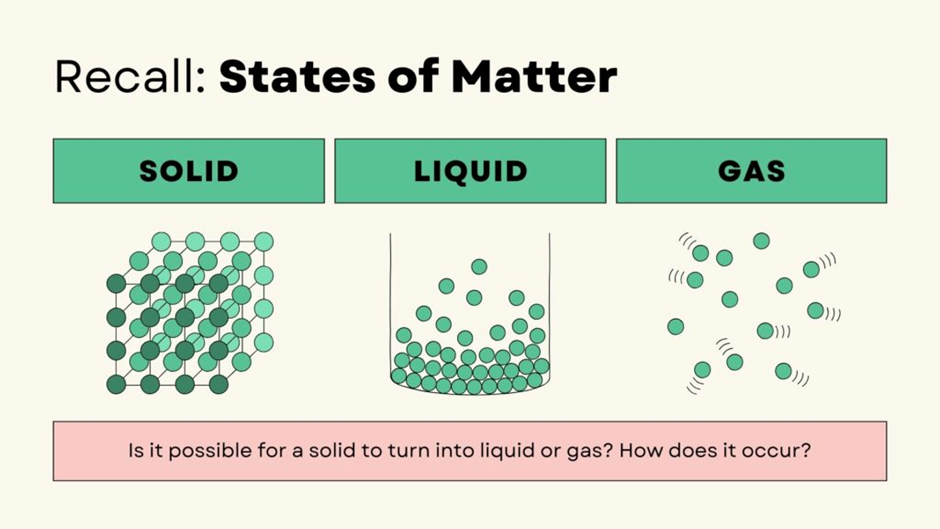
What is the Triple Point?
The Triple Point of a substance is the unique temperature and pressure at which it exists in all three phases (solid, liquid, and gas) simultaneously. At this precise condition:
✅ Water boils (turns into gas)
✅ Water freezes (turns into ice).
✅ Water remains in its liquid state
✅ All three states exist together in perfect balance!
For water, the Triple Point occurs at 0.01°C (273.16K) and 611.657 pascals (0.00604 atm) of pressure. This pressure is much lower than Earth’s normal atmospheric pressure at sea level (1 atm).
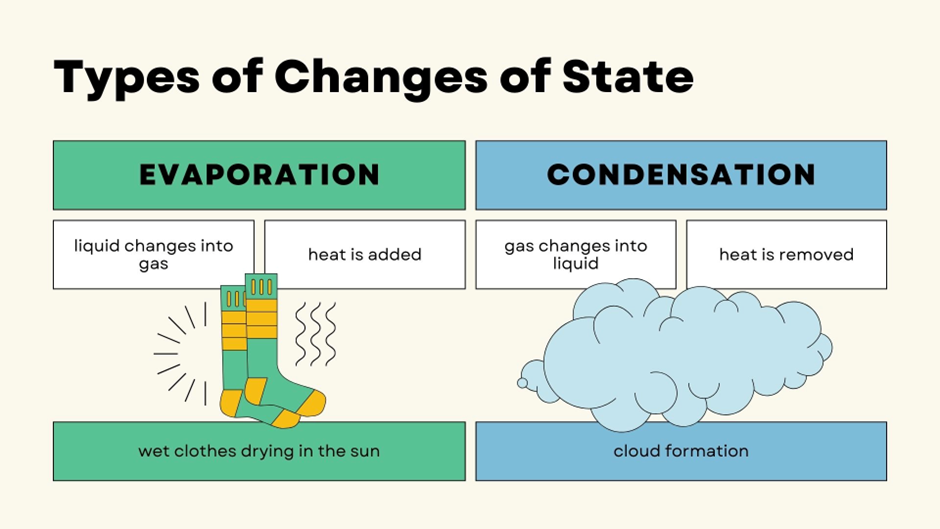
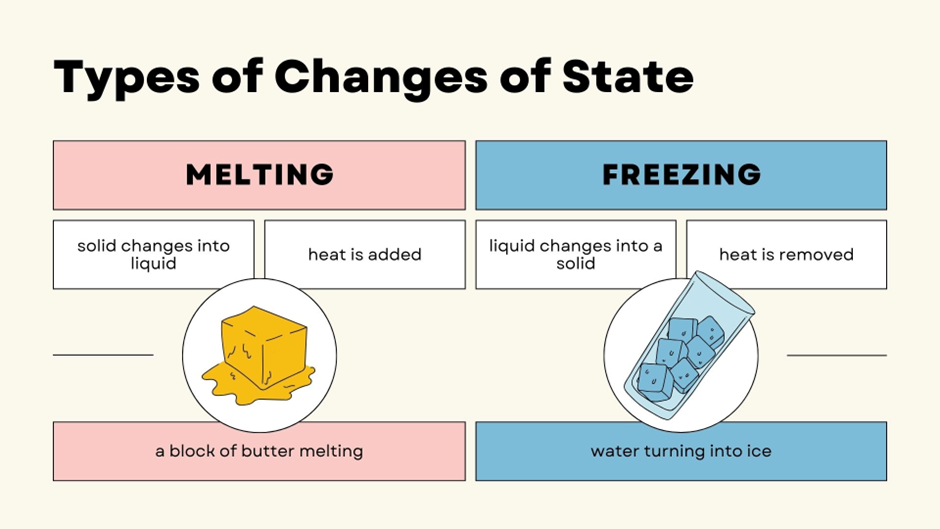
How Does Water Boil and Freeze at the Same Time?
Under normal conditions, we are used to seeing water boil at 100°C and freeze at 0°C. But at the Triple Point, the unique combination of low pressure and temperature creates a balance where the molecules of water exist in all three states at once.
Here’s what happens at the Triple Point:
✅Ice Melts into Water – The temperature is just above freezing, allowing solid ice to become liquid.
✅Water Evaporates into Gas – The pressure is so low that liquid water can turn into vapor easily.
✅Gas Condenses into Liquid and Freezes Again – The system continuously shifts between these states, with some vapor condensing back into liquid and some liquid freezing into ice.
This phenomenon can be seen in a vacuum chamber, where the pressure is reduced to 0.00604 atm.
Scientific Explanation of the Triple Point
Understanding Phase Diagrams
Every substance has a phase diagram, which shows the relationship between pressure, temperature, and state of matter.
📌 The Triple Point is the spot on the phase diagram where the three states (solid, liquid, and gas) intersect.
📌 If you lower the pressure below the Triple Point, water sublimates (turns directly from ice to vapor, skipping the liquid phase).
📌 If the pressure is above the Triple Point, water behaves normally—freezing at 0°C and boiling at 100°C.
This means that for water to boil and freeze at the same time, it must be at exactly 0.01°C and 611.657 pascals of pressure.
Where Can We Observe the Triple Point?
1. Laboratory Vacuum Chambers
✅Scientists create the Triple Point of water in controlled vacuum chambers.
✅A small amount of water is placed in a low-pressure environment, leading to simultaneous boiling and freezing.
2. High-Altitude Environments
✅ On Mount Everest, where atmospheric pressure is much lower than at sea level, water boils at around 70°C instead of 100°C.
✅ In even lower-pressure environments, water could reach the Triple Point naturally..
3. Space Exploration
✅ In space, water behaves differently due to extreme vacuum conditions.
✅ If exposed directly to the vacuum of space, water can boil and freeze simultaneously because of the lack of atmospheric pressure.
Real-Life Applications of the Triple Point
1. Temperature Calibration
✅ The Triple Point of water is used to define the Kelvin temperature scale (273.16 K).
✅ Laboratories use it to calibrate thermometers with extreme precision.
2. Scientific Research
✅ Studying the Triple Point helps scientists understand phase transitions, pressure effects, and extreme conditions found on other planets.
3. Industrial & Engineering Uses
✅Cryogenic engineering and space technology utilize phase diagrams for designing systems that work under extreme temperatures and pressures.
How Can You See the Triple Point at Home? (DIY Experiment)
While achieving the exact Triple Point of water at home is difficult, you can get close with a simple experiment!
What You Need:
✔ A vacuum-sealed jar or syringe (without the needle).
✔ A small amount of water.
✔ A syringe pump or vacuum pump.
Steps to Follow:
- Fill the syringe with water – Just a few drops will do.
- Seal the syringe and create a vacuum – Pull the plunger back to lower the pressure inside.
- Observe the changes – The water will start boiling at room temperature due to reduced pressure.
- Some of the water will freeze – Because the evaporation process cools the remaining liquid.
- You’ve created a mini version of the Triple Point! 🎉
Mind-Blowing Facts About the Triple Point
🔥 Did You Know? The Triple Point of carbon dioxide (CO₂) is used in dry ice production!
🌍 On Mars, water reaches its Triple Point much more easily due to lower atmospheric pressure.
🚀 NASA and ISRO study Triple Point effects for designing life-support systems in space.
Conclusion
The fact that water can boil and freeze at the same time is one of the most fascinating concepts in physics. This happens at the Triple Point, a unique combination of temperature and pressure where all three phases—solid, liquid, and gas—exist simultaneously.
From scientific research and space exploration to practical engineering applications, the Triple Point has many real-world implications. And while we may not see this phenomenon in our daily lives, it is a crucial part of understanding the laws of thermodynamics and phase transitions.
So next time you heat water to boil or freeze it into ice, remember that under the right conditions, it could be doing both at the same time! 🚀
FAQs About Water Boiling and Freezing at the Same Time (Triple Point)
1. What does it mean when water boils and freezes at the same time?
It means that under a specific combination of temperature (0.01°C) and pressure (611.657 pascals or 0.00604 atm), water can exist in all three states—solid (ice), liquid (water), and gas (steam)—simultaneously. This is known as the Triple Point of Water.
2. What is the exact temperature and pressure for water’s Triple Point?
The Triple Point of water occurs at 0.01°C (273.16 K) and 611.657 pascals (0.00604 atm).
3. Why doesn’t water boil and freeze at the same time in normal conditions?
Under normal atmospheric pressure (1 atm), water freezes at 0°C and boils at 100°C. The Triple Point requires extremely low pressure (0.00604 atm), which does not naturally occur at sea level.
4. Can I see water boiling and freezing at the same time at home?
Yes! While achieving the exact Triple Point requires specialized equipment, you can try a DIY experiment using a syringe and vacuum pump to lower pressure. This will cause water to start boiling at room temperature, and some of it will freeze due to rapid evaporation.
5. What is the significance of the Triple Point?
The Triple Point of water is used to define the Kelvin temperature scale (273.16 K). It also helps scientists study phase transitions, space exploration, and industrial applications like cryogenics and thermodynamics.
6. Does the Triple Point exist for other substances too?
Yes! Every substance has its own unique Triple Point, where its solid, liquid, and gas phases coexist. For example:
- Carbon Dioxide (CO₂): Triple Point at -56.6°C and 5.11 atm (used in dry ice production).
- Mercury: Triple Point at -38.8°C and 0.165 mPa.
7. Can water reach its Triple Point naturally on Earth?
Not easily! However, in extreme high-altitude locations like Mount Everest, water boils at a lower temperature due to reduced air pressure. In some low-pressure environments like deep caves or Mars, water can naturally reach its Triple Point.
8. What happens if pressure drops below the Triple Point?
If pressure drops below 0.00604 atm, water cannot exist in liquid form anymore. It will either sublimate (turn from ice to vapor directly) or condense from vapor to ice, skipping the liquid phase entirely.
9. Why is the Triple Point important for space exploration?
In space, there is no atmospheric pressure, so water exposed to a vacuum will boil and freeze at the same time. Scientists study the Triple Point to understand how liquids behave in low-pressure environments like Mars or the Moon.
10. Can I experience the Triple Point effect in daily life?
Not directly, but you can notice similar low-pressure boiling effects in high-altitude cooking. Water boils at lower temperatures in the Himalayas or on an airplane due to reduced pressure, though it won’t reach the exact Triple Point.